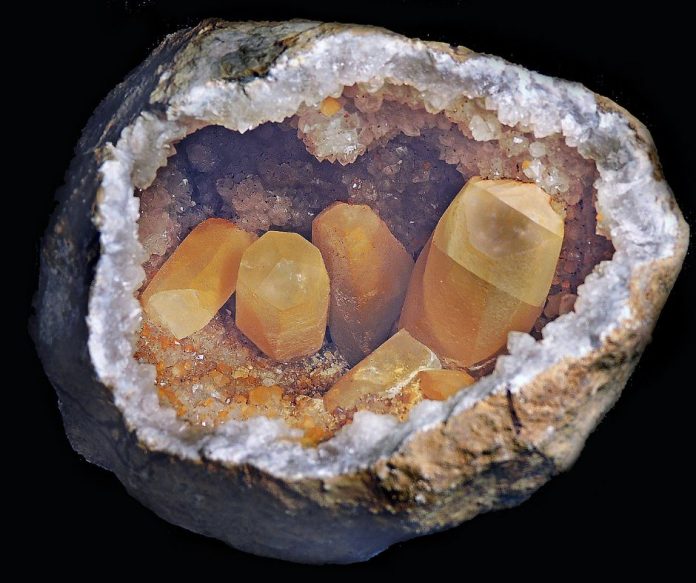
Calcite identification is important because calcite (CaCO3) is found in many crystal forms and colors and is a popular addition to most mineral collectors’ inventory. Here’s a helpful guide to get started identifying!

Crystal Formation & Properties
Calcite crystals form in the trigonal system and their habits vary more than any other mineral. Common forms include dogtooth, tabular, needle-like, pseudocubic and prismatic. With a Mohs hardness of three, rhombohedral cleavage and bubbling in contact with acid, calcite is easily identified.
Calcite dissolves in acid — almost no other minerals do — and can be useful when calcite entombs a more desirable mineral. Soaking the specimen in acid will dissolve the calcite and expose the desirable mineral. This is a common technique used by geode collectors.
Calcites’ colors include white, clear, gray, yellow, green, blue, brown, and black. Some calcite will fluoresce under both short-wave and long-wave UV light. Calcite may be found in caves as stalactites or stalagmites. Calcite and aragonite are polymorphs as they have the same chemical composition (CaCO3) but have different crystal structures.

Calming Calcite
For those that perceive calcite as having healing powers, calcite is said to have the power to cleanse energy, enhance spiritual growth, balance chakras, absorb negative energy and calm the mind.
Calcite Identification
There are over 300 forms of calcite, some more popular than others. Gem-quality calcite is often translucent and is rarer than other mineral specimens. It is colorless, white, gray, yellow, pink, or green. Iceland spar is a clear, colorless, transparent variety of calcite that exhibits strong double refraction or birefringence. It is said the Vikings used Iceland’s spar for navigation.
Calcite is also a component in many rocks such as limestone, marble, and carbonatite. Its name comes from the Greek word calyx which translates to “lime” and relates to being a component in limestone. Calcite is also the mineral component of marble used in sculpture and decorative household use. Carbonatite is important with many uses such as host rocks for fluorspar, rare earth elements, apatite, and vermiculite, as well as a source for calcite.
Countries of Origin

Afghanistan; Argentina; Australia; Austria; Belgium; Bolivia; Brazil; Bulgaria; Canada; China; Colombia; Congo; France; Germany; Greece; Iceland; India; Ireland; Italy; Kosovo; Latvia; Madagascar; Mexico; Morocco; Namibia; Norway; Pakistan; Peru; Portugal; Russia; Slovakia; South Africa; Spain; Sweden; Switzerland; Tanzania; United Kingdom; United States
This story about calcite identification previously appeared in Rock & Gem magazine. Click here to subscribe. Story by Richard Gross and Pam Freeman. Photos by Richard Gross.