
Break out fluorescent lamps to make maximum use of the dark by viewing minerals that glow in vivid colors when black nights envelop us. Explore the phenomenon of fluorescent minerals and glow through the longest nights!

Fluorescent Minerals Defined
When collecting fluorecent rocks, it’s important to understand what gives minerals color and how light moves in waves and comes in different forms depending on the wavelength — infrared, visible, or ultraviolet. We are most familiar with visible light. Ultraviolet (UV) light moves in waves too short for our eyes to detect, but we can see its effects with certain minerals. What appears to be a drab gray rock in visible light may glow a vivid orange or green under UV light. Or a mineral of one bright color under visible light may appear a different color under UV. For instance, green fluorite may turn blue. Other minerals may stay the same color but appear much more intense, as with red ruby. In all cases, with UV light the minerals seem to glow from within, much like an electric neon sign glowing against a night sky.
What Causes Fluorescence?
Some minerals absorb ultraviolet light, which is invisible to us. But they then emit longer, visible light waves which we see as colors. At the atomic level, ultraviolet light causes electrons in some molecules to absorb extra energy and jump to a higher energy level. In falling back to a normal level, they give off that extra energy in the form of visible light. Thus the bright lights we see glowing in the dark.

Thank You, Sir George!
The first person to describe the phenomenon of rocks glowing in the dark under ultraviolet light was English scientist Sir George Stokes, 1st Baronet (1819-1903). Sir George was a physicist and mathematician at the University of Cambridge in England. In 1852, Sir George conducted experiments with fluorite under ultraviolet light. Because he was working with fluorite, he called the optical effect “fluorescence.” This, along with his many other scientific accomplishments, earned him the position of President of the Royal Society and Master of Pembroke College, Cambridge, along with service in the British House of Commons. He was a true polymath or a person with expertise spanning a wide range of subjects.
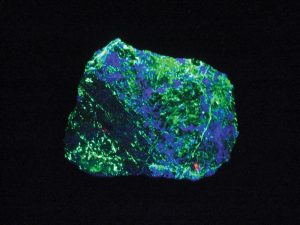
Short-Wave vs. Long-Wave
Ultraviolet light is usually divided into short-wave and long-wave. Most fluorescent minerals are sensitive to short-wave so most fluorescent mineral collections focus on such minerals. But some minerals will change color as you switch from short-wave to long-wave. It’s a good idea to have lamps that allow for both short-wave and long-wave illumination.
Does Every Mineral Fluoresce?
3,600 minerals have been identified, categorized, and named. Only 14 percent, or 500, fluoresce. To build a basic fluorescent mineral collection, seek minerals like calcite, opal, ruby, scheelite, willemite, celestite, hydrozincite, barite, scapolite, aragonite, and halite.
Learning More About Fluorescent Minerals
Good books for a fluorescent mineral collection include Manuel Robbins’ Fluorescence: Gems and Minerals under Ultraviolet Light, Stuart Schneider’s The World of Fluorescent Minerals and Schneider’s Collecting Fluorescent Minerals. You’ll also find many good websites and there’s even a Fluorescent Mineral Society (uvminerals.org) you can join.
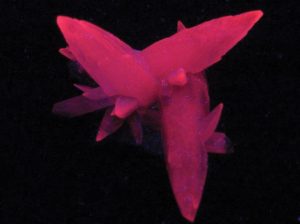
If Working with Fluorescent Minerals, Beware
A warning! Don’t look directly into a fluorescent lamp. While long-wave ultraviolet light is relatively harmless, short-wave ultraviolet light can “sunburn” skin and eyes. Even though protective sunglasses can act as a shield, time spent with ultraviolet lights should be limited. Be safe, not sorry, while enjoying fluorescent minerals.
This story about florescent minerals appeared in Rock & Gem magazine. Click here to subscribe. Story by Jim Brace-Thompson.















