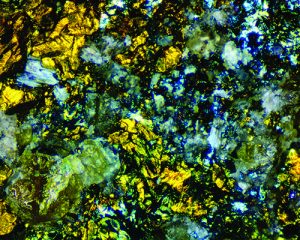
Iridescent rocks are familiar members of the mineral world that we prize such as opal and moonstone. With vivid colors and infinite variations, the beauty of iridescence is indeed in the eye of the beholder. Iridescence is one of the mineral kingdom’s most beautiful displays of color.
Iridescent Opal

The gemstone that is synonymous with eye-catching iridescence is opal, the national gemstone of Australia. Opal is prized for its kaleidoscopic displays of fiery iridescence. It also consists of packed layers of nanoscale silica spheres. Depending upon their arrangement, these layers act as thin films and diffraction gratings to create the distinctive “patchwork” of structural colors known as opalescence.
Sunstone & Moonstone
Two other gemstones that occasionally exhibit iridescence are sunstone and moonstone, both of which are translucent varieties of feldspar minerals. In sunstone, a bright, golden glitter enlivens the stone’s brownish-red body color. This non-iridescent glitter, known as aventurescence, is caused by light reflecting from included microparticles of hematite and goethite. When these particles act as diffraction gratings, the stones exhibit a greenish iridescence.
Moonstone is named for its soft, moon-like, silvery-to-bluish-white sheen. This non-iridescent effect, called adularescence, is due to microscopic inclusions or lamellar twinning planes that diffuse light. These inclusions and planes can sometimes cause thin-film or diffraction-grating interference that creates a delicate blue iridescence. Less common is “rainbow moonstone” with its array of pale cyan, green, and gold structural colors.

Iridescent Quartz
Iridescence also appears in certain forms of quartz. Rock crystal from a locality in India’s Deccan Traps has an intense iridescence, but only on specific crystal faces. Scanning electron microscopy reveals that these faces’ surfaces consist of the periodic ridges and grooves of repetitive twinning. These function as diffraction gratings to create the bright iridescence of “rainbow quartz,” fine specimens sold for thousands of dollars.
Surface coatings of particulate goethite and hematite can produce surface thin-film iridescence in rock crystal, while the interfaces of twinning planes and fracture surfaces can cause internal diffraction-grating iridescence. In botryoidal forms of translucent fire agate, a play of green structural colors sometimes accents the stone’s brownish-red base color, an iridescence caused by coatings of particulate goethite on internal growth-layer interfaces.
Iris Agate
Iris agate exhibits an unusual iridescence. In reflected light, finely banded, semitransparent iris agate has a drab, gray body color. But when backlighted, thin slices cut perpendicular to the banding produce a circular rainbow of structural colors, called an “iris,” due to the diffraction-grating effect of microscopic growth layers.
The brilliant iridescence on botryoidal goethite specimens is due to microscopic surface layers of “turgite,” a mix of goethite and hematite that causes thin-film interference. Turgite films also create iridescence on fracture surfaces of flint and other types of microcrystalline quartz.
Rainbow Obsidian
Aptly named rainbow obsidian displays both internal and surface iridescence. Obsidian, a volcanic glass, typically has inclusions of mineral particles or gas bubbles that act as diffraction gratings to produce internal iridescence; weathering produces thin-film, surface iridescence.
Surface coatings and internal twinning planes occasionally create iridescence in such minerals as calcite [calcium carbonate, CaCO3], fluorite [calcium fluoride, CaF2], and several garnet-group members, notably andradite [calcium iron silicate, Ca3Fe2Si3O12].
Satin Spar
Satin spar, the fibrous variety of gypsum [hydrous calcium sulfate, CaSO4·2H2O], can exhibit both chatoyancy and iridescence. Satin spar’s densely packed, longitudinal fibers scatter light to create chatoyancy, a soft, glowing, white band that shifts as the viewing angle changes. When properly spaced, these fibers become diffraction gratings that cast a soft, iridescent cyan.
Iridescent Shells
Abalone shells and certain other seashells are also often iridescent. These shells’ nacre linings consist of tightly packed layers of hexagonal platelets of aragonite, the orthorhombic polymorph of calcium carbonate. These act as diffraction gratings that impart a soft, pink-and-green iridescence to the shells’ inner surfaces.
Fossilized shells of nautilus-like ammonites, notably those from the Bearpaw Shale formation in Alberta, Canada, show intense magenta-and-green structural colors due to the diffraction-grating effect of their aragonite layers.
Iridescent fossilized ammonites are sold commercially as “ammolite” and worn as pendants. Most pearls have uniform, non-iridescent, white, or cream body colors. But when nacre layers cause diffraction-grating interference, pearls can appear multicolored with an overtone or “orient” of soft, pink-and-green iridescence.
Synthetic Iridescence
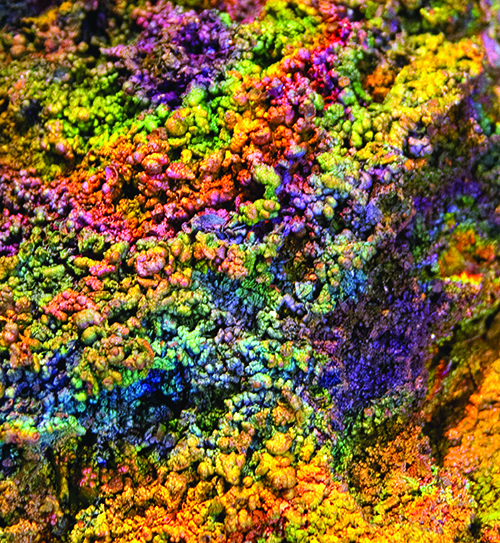
Certain human-made materials are also iridescent. Most ancient glass, and even modern glass that has been buried for a few decades, become iridescent when weathering separates its surface into thin films.
Mass production of synthetic, iridescent materials began in the early 19th century when European ceramic artists began mixing silver and copper oxides into glazes for fine china. High-temperature firing reduced these oxides to metal microparticles that acted as diffraction gratings. In 1894, the American jewelry and decorative-art designer Louis Comfort Tiffany patented a similar process to manufacture his famous line of iridescent “Favrile” glass.
Synthetic Bismuth
The intensely iridescent, synthetic bismuth crystals are often seen in rock shops and at gem-and-mineral shows are made by cooling molten bismuth slowly so that it solidifies into “hopper”-shaped crystals.
The solidifying bismuth’s surface reacts with atmospheric oxygen to produce thin, bismuth-oxide coatings and resulting in thin-film interference. Even slag, the waste produce of metal smelting, can be iridescent when concentric surface layers of silica produce thin-film interference. Iridescent smelter slag is occasionally fashioned into jewelry.
CDs and DVDs
The playing surfaces of polycarbonate-plastic CDs and DVDs are another example of human-made iridescence. These disks are engraved with thousands of nanoscale grooves and ridges arranged in spiral tracks and coated with a bright, metallic finish. These highly reflective, diffraction-grating surfaces create a wide range of iridescent colors. Material scientists have recently developed lacquers and paints that impart iridescence to such products as cell phone cases, fingernail polish, and automotive finishes.
Artificial Iridescence
But not all structural colors are iridescent. One example is the kaleidoscopic “patchwork” patterns seen in photomicrographs of thin sections of rock. These structural-color images of a rock’s microcrystals are caused by cross-polarized light reflecting from, or passing through, birefringent mineral crystals. Because these structural colors are mineral-specific, they help to identify the rock’s mineral components.
Artificial iridescence can be readily imparted to non-iridescent mineral specimens, and iridescence’s intensity can be enhanced on those that are naturally iridescent. The colorful, iridescent “peacock ore” sold in rock shops as “bornite” is usually golden chalcopyrite that has been oxidized by acids or rust-removal compounds to produce an artificial iridescence. Bleach can also induce an attractive iridescence to disk-shaped pyrite “suns.”
Additionally, vapor-deposition processes can inexpensively emplace metal-oxide coatings that, through thin-film interference, transform common rock crystal into artificial “rainbow” quartz.
This story about iridescent minerals is part of a two-part series that previously appeared in Rock & Gem magazine. Click here to subscribe. Story by Steve Voynick.