Zinc metal comes in a variety of lovely mineral species but not in a pure state. It ranks as the fourth most abundant metal after iron, aluminum, and copper. But unlike copper, iron, lead, and some other metals, pure zinc is never found in a natural state. It is so reactive scientists had a heck of a time isolating pure zinc so they could study it.
Zinc Metal: Finding Its Pure Form

Zinc has been in use for well over 5,000 years, but it was never found in pure form until 1746, thanks to zinc’s disappearing act! Scientists tried to isolate it as zinc readily forms compounds but can’t stand being alone, so it forms compounds with almost anything that comes along. The problem with zinc is it is very chemically active. Alchemists and later metallurgists and scientists could produce all sorts of chemical reactions involving zinc. Still, as soon as it was isolated, it would combine with something at hand, usually oxygen.
Zinc was so elusive scientists were really stymied to produce it in pure form. Workers would perform a perfect chemical operation to free up zinc only to open their furnace or container and not find anything, no zinc, only unwanted residue. Where was the zinc?
Zinc Metal: Right In Front of Us
What’s funny is the zinc was right there. The residue weighed less than the original material, so it seemed to have disappeared. To find the missing zinc, all the scientists had to do was look up the chimney. There it was! The zinc had vaporized from the heat of the reaction and drifted up the chimney, combined with oxygen, and sublimated on the walls of the chimney as white zinc oxide. Mystery solved! But the white powder was still not pure zinc.

What’s interesting today is that you can buy bright yellow-green zinc specimens that have formed in the chimney or a furnace. The crystals are lustrous, richly colored, fluorescent, and are spiky in form. Maybe that’s why its name comes from the German word zinke, which means tooth!
The element zinc is so common and so chemically active that there is a large variety of zinc-rich mineral species. I seriously doubt there is a general mineral collection anywhere in the entire world that lacks a variety of zinc minerals.

Useful Zinc
Just because metallurgists and scientists had trouble isolating zinc metal does not mean it was not useful. In fact, by 3,000 B.C., metallurgists produced a golden alloy of copper and zinc we call brass. They even used the brass for coinage. They learned to control, to some extent, the copper-zinc ratio to achieve different versions of the alloy, influencing its brassy color and hardness. This state was possible because the two metal elements, copper and zinc, are substitutional and can replace each other easily.
If you look at the Periodic Table of Natural Elements, you’ll note that copper, atomic number 29, is followed by zinc, atomic number 30. That means zinc has just one more proton than copper in its nucleus and has one more electron to maintain electrical balance. As a consequence, these two atoms are almost the same size with only slightly different atomic weights. They also have the same outer orbital valence of negative two. For brass production, this is very important because these two elements have no trouble replacing each other in an atomic structure. Zinc readily substitutes for copper atoms in the atomic structure to form the alloy brass. The amount of zinc substitution determines the properties of the final product.
This trait of zinc metal substituting for copper is used in the process of making bras called cementation. Copper and zinc compounds are melted in a furnace, and as the zinc atoms are released, they immediately combine with copper atoms to form brass.

Extracting Zinc Metal
Early metallurgists and chemists had a real problem extracting pure zinc metal when free ions of zinc are heated and turn to a vapor, rise then cool, and sublimate to a solid state. This leaves the experiment going up the apparatus’s chimney or reverberatory furnace, combining with oxygen as it rises. The gas cools, forming solid white zinc oxide crystals on the chimney walls. In early chemistry, this material was called calamine and could be used to make copper-zinc alloy, brass.
Though metallurgists and scientists could produce brass by controlling temperatures and regulating the melt’s content, they could not produce pure zinc. It remained for German scientist Andre Markgraaff to isolate the metal in 1746. By that time, the production of brass was well advanced using both primary and secondary zinc minerals.
Since zinc metal is so common on Earth, the range of zinc minerals is broad. The primary zinc sulfide is sphalerite, which is found in huge deposits in America, Australia, and other countries. Wurtzite, another zinc sulfide, is much less common but is sometimes used as a zinc ore.

Sphalerite
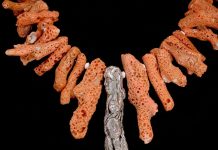
Though sphalerite is readily available, collectors are far more interested in the secondary zinc minerals, which range from rare to plentiful and colorful. Sphalerite is certainly a fine mineral to collect and is normally thought of as a dark resinous mineral close to black, which is due to iron in its structure. Lacking iron, it can be transparent and any gemmy color from red to green to yellow and colors in between. It has a high refractive index and luster.
Sphalerite is the major ore of zinc and is found in deposits all over the world. The U.S. has many productive hydrothermal and contact metamorphic deposits. Of all the productive deposits, two were particularly well-known and produced collector specimens: Franklin, New Jersey, and the Tri-State area of Oklahoma, Kansas, and Missouri.
The Tri-State area produced countless specimens of sphalerite associated with galena, chalcopyrite, calcite, and fluorite, which are still seen on the market. The Franklin-Sterling Hill deposits were, until the 1950s, major producers of zinc in franklinite, zinc silicate willemite, and zinc oxide and zincite as the major ores. The Jersey deposits are universally known as major sources of fluorescent minerals.

Collecting Secondary Minerals
Sphalerite is a fine mineral to collect, but most collectors are more attracted to the common secondary minerals resulting from sphalerite weathering. Everyone loves smithsonite, aurichalcite, rosasite, and hemimorphite, as these have been found in quantity. Much less often are rare zinc minerals like tephroite, koetigite, hopeite, phosphophyllite, even franklinite, and legrandite.
Franklinite
Franklinite is not hard to find at Sterling Hill or Franklin as it occurs as black, rounded grains in the ore. Good crystals, on the other hand, are hard to find now. The better crystals are partially embedded in the white marble, often with grains and small crystals of willemite and massive red zincite. Sharp black octahedrons an inch or more on edge are rare now. One caution to note is that franklinite crystals are very brittle and easily chipped and broken. A very common practice among miners and collectors of damaged franklinite in the old mining days was to repair broken crystals with plaster, paint the repaired area black, and sell the specimen.
Hopesite
A really rare zinc mineral is hopesite, a zinc phosphate hydrate. Originally found as small crystals in Germany, wonderful specimens were encountered in one African locality originally named Broken Hill, North Rhoddesia, now Kabwe, Zambia. When discovered, Rhodesia’s Broken Hill resembled the famous Australian locality and was so named. Hopeite was found here in lovely small white crystals in tight clusters. The crystals are usually no more than a half-inch long, but large specimens were once common 70 years ago. The last Kabwe hopeite I saw available were small brown orthorhombic crystals stained with iron oxide.

Phosphophyllite
Phosphophyllite is equally rare, found originally at Hagendorf sud, Bavaria, as crystals to an inch or so. The finest phosphophyllite is an amazing six-inch twinned crystal of gorgeous pale blue color on a matrix found in a mine in the Potosi district, Potosi Department, Bolivia. That source has not produced anything in the last couple of decades. Naturally, this six-inch beauty passed through a number of collector’s hands including David Wilber, who allowed me to photograph it.
Minerals from Mina Ojuela, Mapimi, Mexico
The famous silver deposit Mina Ojuela, Mapimi, Mexico, has produced a wonderful suite of zinc minerals, many of them in quantity! They are hemimorphite, adamite, legrandite, and koettigite. This last mineral is uncommon but available. Koettigite occurs here in small needle-like crystal sprays of gray color on a brown iron oxide matrix. Sprays up to two inches long and an inch across are very distinctive.
The hemimorphite is found as snow-white or iron-stained crystals to three inches to four inches long in diverging sprays. Along with adamite, this mineral, a green zinc arsenate, is the more abundant zinc species found here. Vast quantities of both these minerals were mined after World War II and are still for sale today. Remember, adamite may fluoresce a bright green.
Of course, the zinc species’ darlings are smithsonite, especially from Tsumeb and the Kelly mine, N.M., and legrandite from Mapimi. Legrandite’s bright yellow crystals are found in sprays, rounded spherules, and fans. They are usually an inch or two long, their bright yellow contrasting nicely with the brown matrix.
So, while you are at shows looking for minerals, think zinc, and maybe you’ll find a rare hopeite, an uncommon koettigite, a nice franklinite or willemite, and a colorful adamite or legrandite. One thing you won’t find is a specimen of pure zinc metal. They do exist, but only in a laboratory, or maybe in chimneys and on the exterior of your galvanized steel garbage can, thanks to Luigi Galvani and his zinc experiments. But that’s another story!
This story about zinc metal previously appeared in Rock & Gem magazine. Click here to subscribe. Story by Bob Jones.