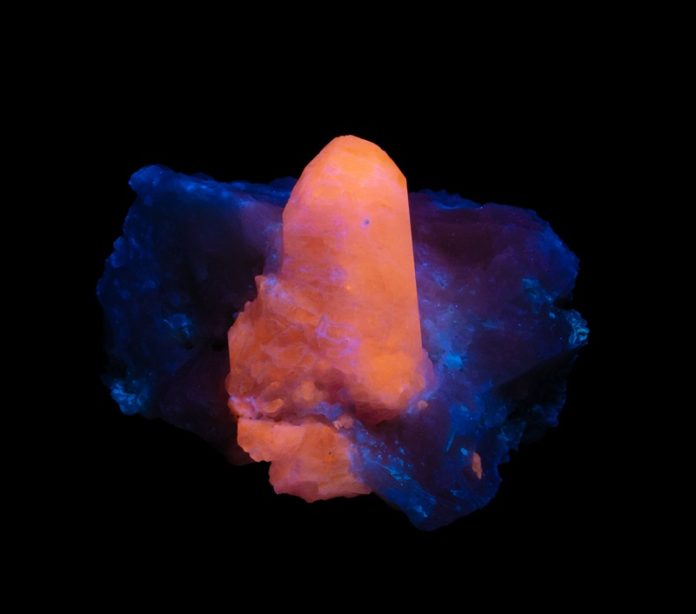
UV rocks are fluorescent minerals and gems whose amazing beauty is on full display when exposed to ultraviolet light. These fascinating rocks have been studied for hundreds of years and are just now beginning to be known. Here’s our guide to these beautiful gems…
Henning Brand & Phosphorus

The man knelt before the glowing waxy mass, believing it held magical powers because it glowed so brightly in the dark. He clasped his hands in reverence believing he had discovered a new power, a power that caused light to appear for no known reason. With this discovery, he went on to fame and fortune, invited by kings to display his magical discovery in court. Wealth and fame were heaped on him and his fame spread throughout the land.
Fairy tale? Indeed not! This happened to the alchemist Hennig Brand when he discovered the element phosphorus in 1669. Searching for the magic elixir to change base metals to gold, Brand decided to try some experiments with human body fluids. Using urine, he boiled and distilled and boiled again until the residue glowed in the dark. The study of luminescence had already started but Brand’s discovery added a great deal of interest and stimulus to the phenomenon. Since then, luminescence has given others a chance at wealth and fortune. It has also produced another fascinating aspect of the gem and mineral hobby, collecting fluorescent minerals and gems.
What is Luminescence?
What is the phenomenon of luminescence? Why do some natural substances glow under certain conditions of lighting and heat, even continuing their glow for hours or days after the conditions have changed?

Luminescence is usually described in two terms, fluorescence and phosphorescence. Fluorescence is used to describe substances, including UV rocks, gems and minerals, which glow when exposed to some form of electromagnetic radiation, including light waves. These days the radiation is usually supplied by an inexpensive long-wave or short-wave ultraviolet lamp. Phosphorescence is the term used to describe substances that continue to glow after the lamp is shut off.
Luminescence has broad applications in many fields including advertising, interior decorating, amusement park rides, posters and billboards, dance halls and nightclubs. The list seems limited only by the user’s imagination. In each case, a substance is exposed to ultraviolet radiation and a bright, seemingly magical glow appears. Colors seem more penetrating and vibrant, and an aura of mystery is enhanced by ultraviolet use.
UV Rocks: Radiation in Mineralogy & Lapidary
The use of ultraviolet radiation in mineralogy and lapidary work has been popular for many years. Over three hundred different mineral and gem species show some response to ultraviolet lamps. Dull, drab, lifeless appearing rocks leap into lighted life under the lamp. Gems of vibrant color seem more vibrant under the lamp, just as they seem to come alive in sunlight, which indeed they do. This is the result of ultraviolet radiation coming from the sun. For instance, when willemite and fluorite are exposed to sunlight they may glow brighter or more intensely. Red spinels and rubies are gems that glow a brighter color on exposure to sunlight. Those invisible ultraviolet rays are the reason. The same rays, and others, are produced by modern ultraviolet lamps.
Seeing a Magical Glow
Hennig Brand wasn’t the first to see a magical glow from a solid substance. An Italian bootmaker named Cascariolo had, in the early 1600s, roasted some barite which, when exposed to sunlight, glowed in the dark.

One of the earliest scientific investigations of the phenomenon of fluorescence was carried on by Sir George Stokes. Stokes worked primarily with fluorite, which occurs abundantly in England. He studied fluorescence from the standpoint of what radiations caused minerals to become excited and where the visible light came from. The result is Stokes’ Law – the wavelength of light coming from a substance is always of longer wavelength than that used to excite the substance. This is evident to mineral collectors who realize that a lamp giving off long wavelengths does not excite as many minerals as lamps of short-wave radiation.
Neither long or short-wave radiation is visible to the unaided eye. When a person sees a fluorescent UV rock, mineral or gem for the first time an immediate reaction is WHY?

Briefly, the invisible radiation coming from the long or short-wave ultraviolet lamp is causing a disturbance in the atomic structure of the mineral. The classic arrangement of an atom is a nucleus surrounded by warming clouds or rings of electrons. These electrons stay put because they possess a certain amount of energy. The ultraviolet lamp force-feeds more energy into these electrons. If they absorb it, they move from their normal positions. Their return to normal, or the movement of other electrons to fill the gaps created, causes a release of the “stuffed” energy as visible light, the magical glow you see. If the visible light stops when the lamp is shut off we call this fluorescence. If it continues after the lamp goes off we assume some electrons are still searching for spots to fill and, once found, release the light gradually as phosphorescent light.
What Causes Fluorescence?
Scientists suggest two causes of fluorescence and phosphorescence, trace amounts of impurities or structural defects. Pure zinc silicate, willemite, doesn’t fluoresce but add a trace of manganese and the willemite glows a brilliant green. Add manganese to calcite and it will glow a brilliant red. Traces of lead cause several minerals to glow a bright yellow or red. Fluorite may fluoresce as a result of any one of several rare earth elements.

Structural defects are not a frequent cause of fluorescence but fracture lines, internal displacement of atoms and inclusions are just a few of the possible defects. Fluorite from southern Illinois is a readily available mineral. Much of it contains minute spherules of petroleum which glow yellow under the ultraviolet lamp. The oil may orient along cleavage planes or simply be scattered throughout. The effect is, of course, a spotty but interesting fluorescence.
Any given mineral may or may not fluoresce. It depends on the imperfection or activator present. Of the known fluorescent gems and minerals only a few deserve the attention of a casual fluorescent collector. They would be the brightest or those showing some uniqueness.

Understanding Phosphorescence
Phosphorescence is an unusual property and deserves more attention. Recent applications in science have given phosphorescence greater significance. Remember that phosphorescence probably results from wandering electrons within the structure of the gem or mineral. Should an abundance of these free electrons become trapped, they may not give up their visible light energy readily. Scientists have found, however, that if the substance is gently heated, the trapped energy may be released resulting in a soft glow of light.
Fluorite shows this property in varying degrees. Gently heated, the fluorite may glow a pale green. Scientists have discovered that careful measurement of the amount of energy being released from organic material when heated can offer a measure of the material’s age. This adds a new dimension to determining the age of the earth’s fossils.
Ultraviolet Equipment
Many mineral and gem materials show phosphorescence. One of the most unusual is the bright pink fluorescent calcite from the mercury deposit around Terlingua, Texas. Its afterglow is a brilliant blue under a short-wave lamp. This mineral has been shown to phosphoresce as long as fifty thousand hours. That’s about six years! Gem materials like diamonds and the beautiful lavender kunzite may phosphoresce as well as fluoresce.

As a result of unusual properties and different types of equipment and techniques we can use, ultraviolet equipment in mineral and gem identification is growing all the time.
There are many suppliers of long-wave and short-wave lamps but only two manufacturers produce lamps specifically for collectors in some variety and at varying price ranges. This makes possible the opportunity for any collector to use the lamps for fun or profit.
Long-Wave Radiation
Long wave lamps produce radiation very close to visible purple light in the color spectrum. They do not require filtering, though some are, and their radiation is the same as that which penetrates our atmosphere from the sun, the source of your sun tan. This was also the radiation used by Stokes which he passed through clear fluorite cubes and saw a beautiful violet cone of color within the crystals.
Long wave radiation also stimulated the “bologna stone” of Cascariolo in the early 1600s. Until such radiation sources as X-ray, the iron spark gap machine, and the mercury vapor tube came along, solar long-wave radiation was our best source.
Many minerals fluoresce or phosphoresce under long-wave radiation but not as many as under short-wave lamps. Fluorite fluoresces best under long-wave radiation and is often the center theme in a fluorescent mineral display. A number of the carbonate minerals, calcite, aragonite, witherite and cerussite, may respond under long wave light. Some secondary uranium minerals may also fluoresce under long-wave ultraviolet light.
As for gem materials, the long wave lamp may stimulate diamonds, rubies, opal and several varieties of spodumene. Long-wave radiation however does not approach shortwave radiation in its ability to stimulate mineral and gem materials.
Short-Wave Radiation
Short wave radiation can be anywhere from about 200 angstroms long (a unit of wavelength measurement) up to about 3,000 angstroms. Long wave lamps range from about 3000 to 3660 angstroms, a much more limited range. Modern short-wave lamps produce almost all their radiation at about 2537 angstroms which seems to be as ideal as possible within limits of equipment cost.
Short-wave equipment does offer some special problems. The current method of producing short-wave radiation is with a cold quartz mercury vapor tube. A drop of mercury within the tube vaporizes from the electric current passing into the tube and this produces copious amounts of ultraviolet light and visible light. The visible light is the problem, so special cobalt “glass” filters must be used to absorb the white light and pass the invisible short-wave ultraviolet light onto the gem or mineral in your collection. Unfortunately, the special filters are expensive so short-wave lamps are correspondingly costlier than long-wave lamps.
Before the modern mercury vapor lamp, much more expensive, cumbersome and limited equipment was used. Most notable was the iron spark gap machine. Something akin to creating a miniature lightning storm when using this machine, it produced all the smoke, noise, light and ultraviolet radiation you wanted. This was a cumbersome way to work with gems and minerals. One of the most notable uses made of an iron spark gap machine was when two scientists, G.F. Kunz and C. Bakerville, exposed something over 13,000 gems and minerals at the American Museum of Natural History to the spark gap’s radiation, discovering many hitherto unknown fluorescent and phosphorescent minerals and gems. Their results, published in 1903, led others to continue the search for fluorescent materials as well as better sources of ultraviolet radiation.
During the 1920s and early 1930s, the search for an effective ultraviolet source went on, culminating in the development of the mercury vapor lamp, so successfully used now in fluorescent mineralogy. At present a wide variety of lamps of both long and short-wave types, as well as combinations of the two, are available. This makes it possible for even a beginning collector to have at his elbow another useful tool for broadening his interest and knowledge in mineralogy or lapidary.
7 Fluorescent Minerals & Gems
There are many fluorescent minerals and gems that the average collector might add to a mineral or gem collection. Here are a few significant examples that must be mentioned in detail.
1. Fluorite
Fluorite, whose name is the root of the science of fluorescence, has been used since ancient times as a decorative stone. During Pliny’s time in the Roman Empire, cups were carved from it. Earlier, the Egyptians may have used it as a decorative material.
In the 1500s we know it was used as a flux, helping to lower the melting point of certain ores for the extraction of metals. The users didn’t understand the chemistry of their actions but were successful nonetheless. We still use huge tonnages of fluorite as a flux. During the mining of fluorite for this purpose, great numbers of magnificent fluorescent specimens have been recovered.

Fluorite’s most common fluorescent color is a brilliant blue, best under a long-wave lamp. Shades of purple, brown, yellow and even red are possible but the brilliant blues are usually chosen to represent this mineral in excellent fluorescent collections. Not all fluorite will fluoresce. The yellows and browns of the Midwest are petroleum-activated. The brilliant blues contain several rare earths as activators. Fluorite does not normally phosphoresce, though we have already alluded to the soft green response of the variety, chlorophane, which glows when gently heated.
2. Willemite
The fluorescent mineral with the widest application, whether artificially produced or found naturally, is willemite. Natural willemite from a unique deposit at Franklin, New Jersey glows a brilliant green under a short wave lamp. It may also phosphoresce for hours. Again, a bright green. The willemite of Franklin is found intimately associated with an equally brilliant red fluorescing calcite, a most spectacular combination. The activator in both minerals is a trace of manganese. Most large public displays contain huge masses of this zinc ore as the more significant part of the display.
Scientists from all over the world have studied this unique deposit and a prolific suite of minerals, about thirty of which fluoresce, have been identified and described. No wonder Franklin, New Jersey was declared “Fluorescent Mineral Capital of the World” in 1968. This was done by a special act of the New Jersey State Legislature. The accessibility of such a unique deposit, the presence of two fine museums, collecting spots that still yield, plus the fact that one part of the ore body is still being mined, make Franklin the most frequented mineral deposit in the entire northeast.
3. Benitoite
California yields many fine fluorescent minerals but none is so fine as the rare blue gem benitoite. Benitoite is found nowhere else except in the remote headwater region of the San Benito River in San Benito County. It occurs as bright blue gem crystals in a white natrolite matrix. The mineral has been faceted into beautiful stones, rarely exceeding a few carats. Under the short wave lamp, these UV rocks are a brilliant, vibrant blue, which, against the light matrix seems even more intense.
4. Calcite
Probably the most common of the fluorescent materials would be the calcite group. I say “group” because calcite and calcium carbonate, occur in so many forms and ways. Under either the long or short-wave lamp, calcite may show any one of a dozen responses. It may be white, green, red, blue, pink, lavender, orange, yellow and shades of anything in between. It may or may not phosphoresce and the phosphorescence may not be the same color as the fluorescence. Since calcite is quite soluble in weak acids it may permeate a host of rocks and minerals offering beautiful fluorescent patterns in a wide variety of places.

The cause of fluorescence in calcite can be as varied as the color response. Manganese, rare earths, lead, uranium salts and organic material may all be a cause. The collector should just be grateful for the large number of interesting responses possible. The most unique of the calcites would be the Terlingua, Texas material. The bright red calcite from Franklin is also popular. People who live in milestone regions have their preferences. Cave formations often show a beautiful dreamy to yellow glow, usually with some degree of greenish phosphorescence following the fluorescence. It is not uncommon for tourist cave owners to install a fluorescent mineral display “in situ” for visitors to see and appreciate the UV rocks.

5. Uranium
On or near the Earth’s surface, deposits of primary uranium ores become weathered. These deposits are most often sought with electronic equipment. However, the secondary material UV rocks formed from weathering will often fluoresce bright green, giving the collector a clue to possible hidden wealth. The secondary uraniums such as autunite, meta-autunite, uranophane, schroeckingerite, uranocircite and others all show a green fluorescence to some degree. The finest deposit of secondary uranium ore ever found was the magnificent micaceous meta-autunite at Mt. Spokane, Washington. Huge groups of brilliantly green fluorescent material were extracted and processed for uranium. Fortunately, the material was so beautiful as specimen material that quantities were saved and are now preserved in collections around the world.
The green fluorescence of uranium salts may also show up in unusual places. They frequently invade such common materials as chalcedony, agate, jasper, calcite and aragonite causing otherwise uninteresting material to become of interest to the collector. The common opal of Nevada, which fluoresces a brilliant green, is an example of a uranium-activated fluorescent mineral.
6. Hackmanite

A most interesting and unique mineral comes from eastern Canada. It is called hackmanite, a sodalite mineral. Under the long wave ultraviolet lamp, it shows a bright salmon pink color. The mineral is normally white. If left under the ultraviolet lamp for some time, hackmanite will take on a deep blood-red to purple color which will remain within the mineral after the lamp is taken away. This is not phosphorescence. An actual color change takes place within the rock itself. The color will remain as long as the specimen is not exposed to bright light. The phenomenon, called reverse photosensitivity, is most unusual and hackmanite demonstrates it best of all.
7. Wernerite
Eastern Canada also produced a magnificent yellow fluorescing mineral called wernerite. Occurring in huge masses, this mineral is also a frequent addition to the larger public displays of fluorescent minerals. No other brilliant yellow fluorescent mineral occurs in such abundance. Since it fluoresces best under a long-wave lamp it can be added to even the least expensive fluorescent mineral display.
These are just a few of the brighter, more common fluorescent minerals. In addition to being beautiful under the lamp, most are also hard enough to be cut en cabochon or sphered. The Franklin, New Jersey calcite-willemite combination is often treated this way. Hackmanite, wernerite and even the softer calcites are worked to improve their brightness or to reveal unusual patterns of fluorescence. The secondary uraniums are much too soft but when they impregnate the harder quartz varieties, lapidary treatment is certainly used. Some adventurous artists have also crushed fluorescent minerals, arranging the different color responses into very beautiful pictures under the lamp.
Industry Uses
Industry goes about the serious business of applying the fluorescence and phosphorescence of materials. For detecting mercury vapors an artificial willemite screen is available.
In original televisions, there wouldn’t be a fluorescent screen to look at without the phosphor coatings inside the picture tube. Electron bombardment of the phosphors containing different rare earth activators gave us color television.
Ultraviolet lamps have also been used for prospecting with considerable success. The prime example of their use was in discovering significant deposits of tungsten ore, usually scheelite, which fluoresces a brilliant blue-white under short wave lamp. When pure, its brilliant blue response is unmistakable. As molybdenum tends to replace the tungsten in scheelite the mineral powellite emerges, with a cream to yellow to white response.
Since molybdenum is an undesirable impurity, ores are evaluated by their fluorescent color. Ultra Violet Products, Inc. of San Gabriel, California produces by government license, a special scheelite fluorescent analyzer useful in the field when scheelite is found.
The short wave lamp can also be used to check the efficiency of recovery in the processing operation revealing fluorescent scheelite in the tailings. This same technique of using an ultraviolet lamp to check tailings for ore values has been used with success elsewhere such as in Franklin, New Jersey.
More directly, the lamp has been used in the cobbing of ore at the Al Hyatt mine, in South Rhodesia. Cobbing is a technique of hand-picking the waste rock from the mined ore. The reverse may also occur, picking rich ore from the valueless bulk.
The Al Hyatt mine is a producer of eucryptite, a lithium orthosilicate that alters from spodumene. Lithium minerals are an important source of that element and are found in relative abundance in some pegmatites frequently associated with gem minerals like tourmaline, triphane and kunzite. Eucryptite has also been recovered at Dixon, New Mexico and a couple of pegmatites in New Hampshire and Connecticut but not in the quantity found at Al Hyatt. Lamps are used to search out the delicate rose-red fluorescent color that distinguishes the ore from the enclosing matrix. In this way, large amounts of commercially profitable ore have been recovered.
Fluorescent Mineralogy: UV Rocks
A second fascinating phase of fluorescent mineralogy is the collecting and study of fluorescent and phosphorescent gem materials.
Diamonds
About 20 percent of diamonds exhibit fluorescence. Many African stones show a bright-blue to blue-white response. However, other colors are not uncommon. Diamonds showing green, orange, yellow, pink, white and even violet or brown fluorescence are not rare. Specimens from Brazil, India and also Arkansas have been found to fluoresce. The cause of fluorescence in diamonds most likely is some structural imperfection rather than an activator. However, manganese may be the cause of the orange fluorescence seen in the rare pink diamonds.
The amazing beauty of fluorescent minerals and gems is reason enough to develop an interest in the hobby. The fine equipment now available at most good mineral shops makes fluorescent collecting a hobby anyone can enjoy.”
Can diamonds be authenticated with the ultraviolet lamp? Hardly! Some diamonds, when heated, will fluoresce green. Diamonds that fluoresce bright blue often show a pale yellow phosphorescence but studies have not established much beyond that.
Emeralds
The very rare gem emerald has been reported as fluorescing a weak green but this is most likely due to escaping visible white light from the lamp. The Chivor, Columbia emeralds may show a fair red response. Synthetic emeralds often fluoresce red.
A technique has been developed to distinguish between the natural red fluorescing Columbian stones and synthetics. Synthetics pass wavelengths of light that are shorter than natural emeralds will pass so a suspected stone is placed with an authentic stone on a photographic plate, exposed to a known amount of radiation and the amount of transmitted radiation is revealed on the plate. This, of course, reveals the authenticity of the suspected stone. Certainly, this technique is more involved than a brief explanation can portray but some idea of the process is evident.

Opals
Opal is probably the most worked stone for amateurs. Common opal with no fire is of interest because it may fluoresce a bright green. The Mexican cherry opal usually does fluoresce. Most of the white, milky fire opal from Australia shows some response under the lamp, though it can hardly be called exciting. The color response is usually dull to fair whitish blue or gray under either a short wave or a long wave. The presence of an ultraviolet lamp masks the fire since there is little white light to be scattered so ultraviolet lamps seem hardly of use where opal is concerned. It is possible to see minute cracks and splits in opal with the lamps due to some shadowing, reflecting or refracting. So, the lamps may serve some small use with opal.
Topaz
Topaz seldom fluoresces, the exception being the yellow-orange crystals from the Ouro Preto, Brazil area. These gemmy crystals almost always fluoresce a good to bright orange under either short or long-wave lamps. These crystals are often heated to achieve a pink color. This causes the fluorescent response to alter toward the red end of the spectrum under a long wave lamp.
Tourmaline
Tourmaline has often been reported to be fluorescent. Only the iron-free variety dravite seems to show any notable response and this is seldom used as a gem material. It is a dull to good yellow under a short-wave lamp.
Zircon
Zircon often fluoresces a dull to bright yellow to orange yellow under short-wave lamps. The fluorescence seems to be due to the presence of hafnium. Heated zircon, again an attempt to achieve a desired color, will also fluoresce but is reported to lose that fluorescence when left under the ultraviolet lamp for some time.
Beryl
There are several varieties of beryl such as aquamarine, goshenite and morganite. But they don’t respond at all.
Sapphires seldom show much response either. Alexandrite may glow a dull red under short waves. The yellow sapphires may fluoresce a very good yellow, the color of the response being paler than the natural color. Synthetic alexandrite may fluoresce red but other synthetics don’t seem to respond. As techniques develop in the synthesizing of stones, it should be obvious that the addition of trace elements would be possible, thus simulating the natural stones more closely.
Ruby
One gem that often fluoresces is ruby. A trace of chrome oxide gives these UV rocks their red color and this also seems to affect the fluorescent response. Even in sunlight the ruby seems brighter and is responding to the ultraviolet light from the sun.

An abundance of synthetic ruby is on the market now, much of it fluorescing a much brighter red than natural stones. Natural stones seldom respond as well under short-wave ultraviolet as do the synthetics so some distinction may be possible. Spinels are sometimes sold as rubies but they are a brighter fluorescent red. There is also a special filtering technique available to distinguish spinels from rubies under the ultraviolet lamp. Colorless spinels may resemble diamonds under the lamp, being a brilliant blue-white. However, these UV rocks never phosphoresce as do most fluorescent diamonds. It is impossible for an amateur to use the ultraviolet response of a gem to identify or authenticate it. There are experts available when needed.
Finding UV Rocks
Any enthusiastic fluorescent UV rocks, gems and mineral collector would certainly want to know where to go to find specimens. Fluorescent gems would seem to be out of the question. However, granites may yield zircon; pegmatites may well yield kunzite or some other gem material, alluvial gravels are certainly worth searching since they may yield a variety of fluorescent material.
As for fluorescent minerals, they may be found almost anywhere. In my experience, using the lamp in any place where rocks are exposed may yield a surprise. Sedimentary deposits can contain fluorescent minerals such as celestite, calcite, aragonite, barite and fluorite.

Granite outcrops, already mentioned as a source of zircon, may show traces of uranium minerals, rare earth minerals, apatite which often fluoresces a dull orange and even some fluorescent feldspars. The coarser-grained pegmatites may also provide any of these accessory minerals. One special type of pegmatite, the lithia pegmatite, is the source of the gemmy tourmaline and spodumene varieties. It may also produce such rare fluorescent minerals as pollucite, herderite and amblygonite, as well as fluorapatite, autunite, meta-autunite, uranophane and other uraniums.
Old mining areas with their waste dumps are a particularly good UV rocks spot to check.
Several ore minerals fluoresce. Many of the secondary lead minerals such as cerussite, lead hillite, mimetite, anglesite and massicot may fluoresce yellow under the short wave lamp. The very common zinc ore sphalerite frequently fluoresces a good orange under either a long or short-wave lamp. Weathering of the waste UV rocks, which usually contain traces of ore minerals, can be particularly helpful in developing fluorescent minerals. The gangue minerals calcite, fluorite and barite may provide large masses of fluorescent material for the collector.

Working around old mining areas requires special attention to safety. Ultraviolet lamps are most effective at night when shafts, pits, poisonous insects, reptiles and other dangers are not evident. Scouting the area in daylight first is recommended. Even better, special equipment for blocking out sunlight is available so the lamp can be used anytime. Incidentally, many organic specimens fluoresce. Scorpions, spiders, reptiles, many types of plants, including fungus and lichens, can be quite bright so a poke with a stick seems the better part of valor when reaching for a fluorescent “something” especially at night.
The amazing beauty of fluorescent minerals and gems is reason enough to develop an interest in the hobby. The fine equipment now available at most good mineral shops makes UV rock collecting a hobby anyone can enjoy. Literature available now is most helpful in getting one started and there is an abundance of specimen material available. Some dealers specialize in nothing but fluorescent minerals. There is even a fluorescent mineral collector’s club. From all this, however, the greatest observation that can be made is the appreciation one gains of nature in seeing another aspect of the world around us revealed under the lamp. This cannot help but add to the personal respect and value one should place on nature at its colorful best. Marion Brown Casperson said it best in the pamphlet, “Fluorescence, What It Is.”
Here is a piece of rock, dull and drab. Its grayness is streaked with brown, with lifeless green and lilac spots of black; it shows but little virtue in itself.
No slightest hint of what it may become. But activated, tuned to magic rays, From nature’s wondrous storehouse, It responds, leaps into brilliance–Its Impassioned glow of colors, gorgeous, Overwhelming to behold. – Marion Brown Casperson
This article about UV rocks was previously published in Rock & Gem magazine. Click here to subscribe. Article by Bob Jones.